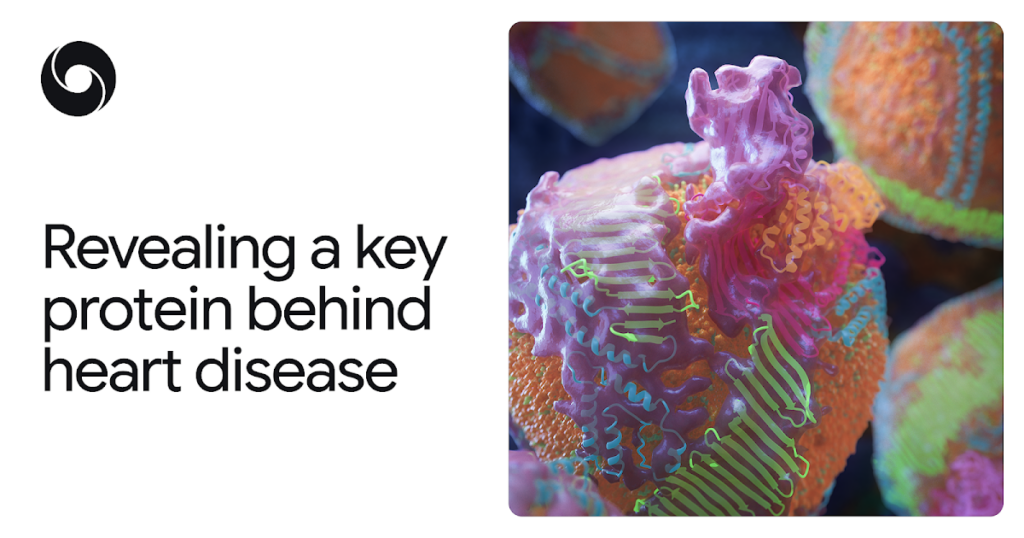
Credit: MolGraphics.com / Zachery Bernsden and Keith Cassidy
A significant scientific advancement, enabled by AlphaFold, has allowed researchers to map the structure of the large protein responsible for the formation of “bad cholesterol.” This breakthrough holds the potential to revolutionize how medical professionals approach the treatment of the world’s leading cause of death.
Identifying a crucial protein involved in heart disease has been a long-standing objective for public health, yet it presented a persistent scientific challenge.
For University of Missouri (Mizzou) assistant professors Zachary Berndsen and Keith Cassidy, this research held personal significance. Both researchers have a family history of heart disease, underscoring the critical importance of their efforts to comprehend and ultimately address this fatal illness.
Berndsen stated, “For 50 years, the scientific community has sought to visualize this protein’s structure.”
The protein in question, apoB100, proved challenging to map due to its considerable size and its intricate interactions with fats and other molecules. ApoB100 serves as the molecular framework for “bad cholesterol,” scientifically termed low-density lipoprotein (LDL).
LDL acts as the primary transporter of fat within the bloodstream and is a significant risk factor for atherosclerotic cardiovascular disease (ASCVD), which is the leading cause of death globally. Uncovering the structure of this vital protein was expected to clarify how “bad cholesterol” contributes to bodily harm, thereby enhancing scientists’ ability to develop prevention and treatment strategies for ASCVD. AlphaFold has been instrumental in this endeavor.
At Mizzou, biochemist Berndsen initially employed cryo-electron microscopy (cryo-EM) to capture images of LDL particles. However, these images lacked the necessary sharpness for atomic-precision mapping of apoB100’s structure. Consequently, Berndsen’s collaborator, physicist Cassidy, utilized AlphaFold to generate atomic-resolution predictions of the protein’s structure. These predicted shapes were then refined by cross-referencing them with the cryo-EM image data.
Cassidy explained that approaching the problem with both cryo-EM microscopy and AlphaFold was key to this breakthrough. He stated, “AlphaFold played a profound role in this discovery, providing the raw material to interpret our experimental structure in a way that was frankly impossible before.”
The model developed showcased the crucial protein of “bad cholesterol” with exceptional detail. It revealed a cage-like shell encasing each LDL particle, complete with a ribbon-like belt that maintains the particle’s integrity in the bloodstream. Understanding this structure creates new avenues for the prevention, diagnosis, and treatment of high cholesterol and ASCVD, potentially leading to therapies that can target LDL with greater precision. The global health implications of this finding are immense.
Although the full application of these findings will require time, the revelation of apoB100’s structure represents a significant milestone, and a deeply gratifying one for Berndsen. He remarked, “It was the first structure I ran through AlphaFold the week it became available, and the first protein I wanted to look at with our two-storey cryo-EM machine. Solving the structure of apoB100 was a dream come true.”